


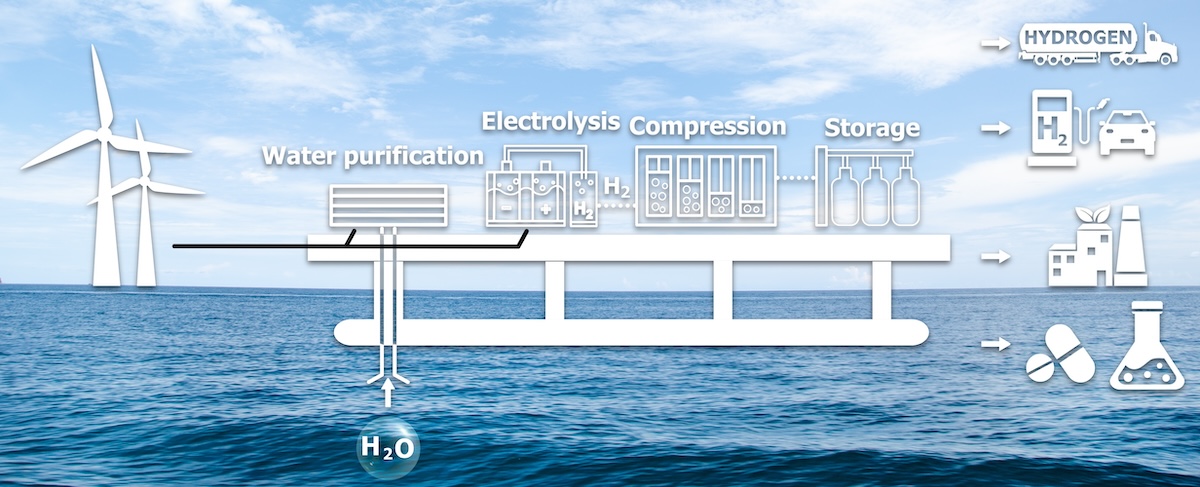
Project developers and engineers planning their energy generation and storage needs have started to turn to water electrolysis for a proven solution with a rapidly expanding technology base. Electrolysers replace fossil-intensive hydrogen sources like steam methane reforming (SMR). By using renewable electricity, water electrolysis eliminates that footprint.
Water electrolysis also allows for localized hydrogen production, reducing transport emissions and pipeline needs. With strategic grid placements electrolysis can further optimize energy efficiency and supply chain reliability.
Water electrolysis separates water molecules (H₂O) into hydrogen (H₂) and oxygen (O₂). This process takes place in a device known as an electrolyser, such as Power To Hydrogen's industry leading hybrid AEM electrolysers. In these systems, hydrogen forms at the cathode and oxygen forms at the anode when electricity flows through water. The electricity for this process is typically sourced from renewable energy, making it a sustainable option with no carbon emissions.
Creating hydrogen through electrolysis has multiple advantages:
PEM electrolysers use a solid polymer membrane as the electrolyte. They produce high-purity hydrogen and respond quickly to changes in power input, making them ideal for pairing with renewable energy sources. However, they rely on expensive metal catalysts, which significantly increases their cost compared to other technologies.
Alkaline electrolysers use a liquid electrolyte, typically potassium hydroxide (KOH), and represent one of the oldest and most widely used electrolysis technologies. Their long track record in industrial settings is due to their low cost and relative simplicity. However, they tend to be larger and slower to respond to fluctuations in power, making them less efficient when paired with variable energy sources.
AEM electrolysers combine the benefits of PEM and alkaline systems. They utilize solid anion exchange membranes and low-cost, non-precious metal catalysts, enabling more affordable hydrogen production with high efficiency and operational flexibility. Power To Hydrogen focuses on advancing this technology, which is already showing promising returns for distributed and renewable-based hydrogen systems.
Solid oxide electrolysers (SOECs) operate at extremely high temperatures and use ceramic materials as the electrolyte. This high-temperature provides the ability to use waste heat in industrial processes. While their efficiency is impressive, they are best suited for large-scale, stationary applications where heat recovery is possible.
.jpeg)
The foundations of water electrolysis go back to the 18th century. Scientists like Alessandro Volta and Michael Faraday laid the groundwork by exploring how electricity affects chemical bonds. Faraday’s laws of electrolysis, established in the 1830s, still form the theoretical backbone of today’s systems.
Faraday’s First Law of Electrolysis: During electrolysis, the amount of chemical reaction which occurs at any electrode under the influence of electrical energy is proportional to the quantity of electricity passed through the electrolyte. - https://byjus.com/chemistry/laws-of-electrolysis/
Faraday’s Second Law of Electrolysis: If the same amount of electricity is passed through different electrolytes, the masses of ions deposited at the electrodes are directly proportional to their chemical equivalents. - https://byjus.com/chemistry/laws-of-electrolysis/
Industrial-scale electrolysis emerged in the early 1900s, primarily for use in the chemical refining industry. But it wasn’t until the rise of renewable energy that water electrolysis started to make today’s headlines as a clean alternative to fossil-fuel-based hydrogen production.
Governments worldwide are introducing tax credits, grants, and hydrogen hub projects to accelerate the deployment of hydrogen infrastructure.
Cost remains a sticking point for many. Electrolysis systems are still more expensive than SMR with carbon capture. But as renewable energy gets cheaper and electrolyser manufacturing scales up, hydrogen from water electrolysis is expected to reach cost parity by the 2030s.
Hydrogen isn’t just for transportation or industry anymore. Meeting regulatory emissions targets will require a greater transition to low-carbon hydrogen. New use cases in data centers, long-haul shipping, and agriculture are expanding the demand.
Efficiency refers to the amount of hydrogen produced for the amount of electricity input, and electrolysis requires significant energy input. Though efficiencies are improving, some energy is lost during conversion and storage. Factors affecting efficiency include the quality of materials used, operating temperatures, and the design of the electrolyser. As technology advances, the efficiency of electrolysers will continue to improve.
At Power To Hydrogen, we focus on developing Anion Exchange Membrane (AEM) electrolysers, the next-generation technology that enables hydrogen production at lower cost and with greater operational flexibility.
Ready to move toward sustainable hydrogen? Contact Power To Hydrogen for a consultation and discover tailored solutions that perfectly meet your needs. Water electrolysis is an essential technology for a sustainable future. Power To Hydrogen provides innovative solutions that make hydrogen production practical, efficient, and affordable. Join the sustainable energy movement today.
