


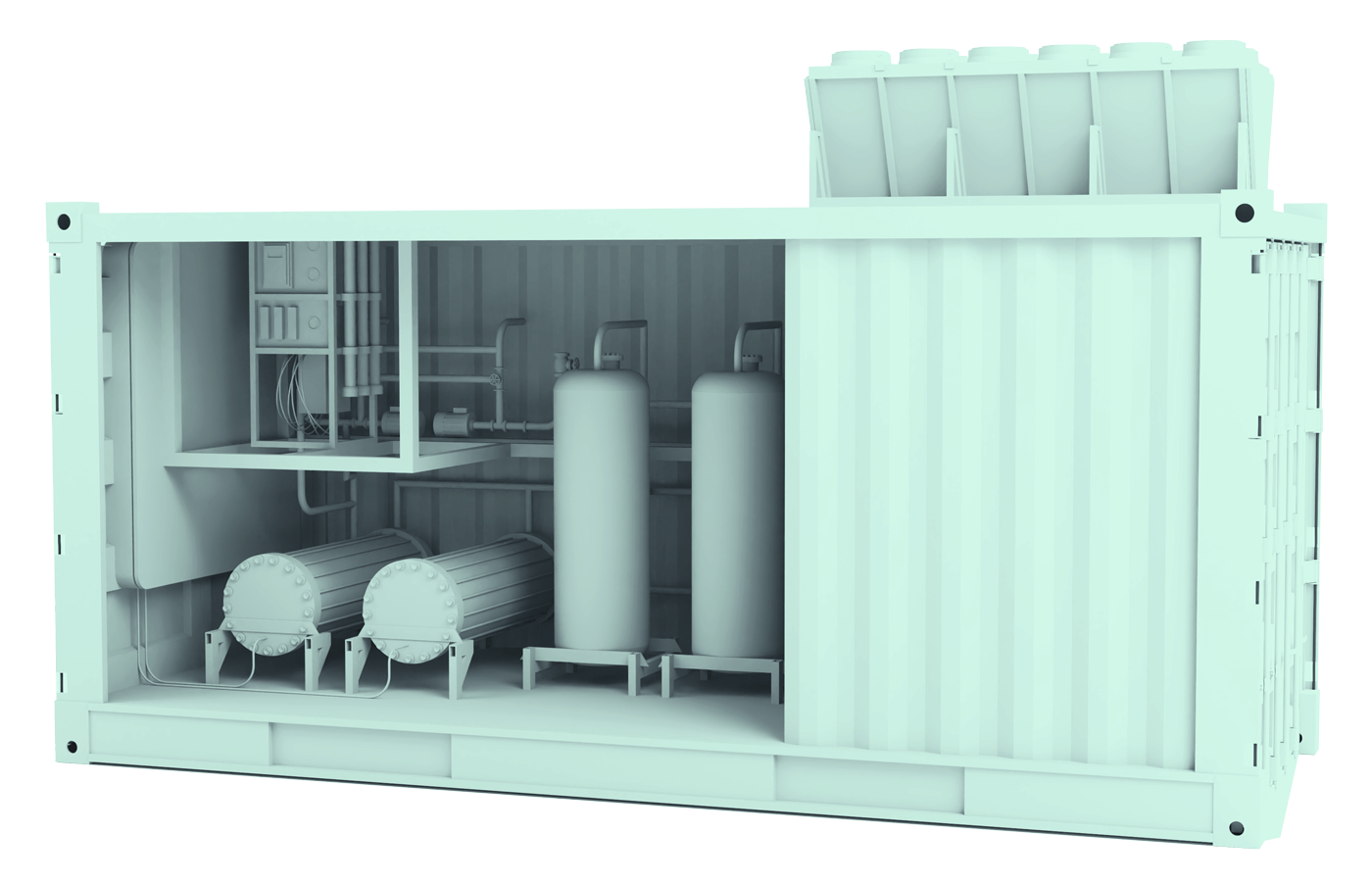
Hydrogen has emerged as a critical energy source in the global effort to reduce carbon emissions. One of the most promising technologies enabling clean hydrogen is the Anion Exchange Membrane (AEM) electrolyser.
AEM electrolysers combine key features of two established systems: the low-cost structure of alkaline electrolysers and the higher performance characteristics of Proton Exchange Membrane (PEM) systems. By leveraging non-precious materials and operating efficiently with renewable energy sources such as wind and solar, AEM electrolysers provide a balanced solution for sustainable hydrogen production.
This guide explores how AEM electrolysers function, what sets them apart from other electrolysis methods, and how Power To Hydrogen’s AEM stack is leading the next wave of clean hydrogen production.
AEM electrolysers split water into hydrogen and oxygen through electrochemical reactions driven by external power sources. Each AEM electrolyser cell consists of an anode and a cathode, which are separated by an anion exchange membrane.
2H2O → O2+4H++4e-
4H2O +4e-→ 2H2+4OH-
4H+ +4OH-→ 4H2O
This ion-exchange process eliminates the requirement for precious-metal catalysts while maintaining high efficiency.
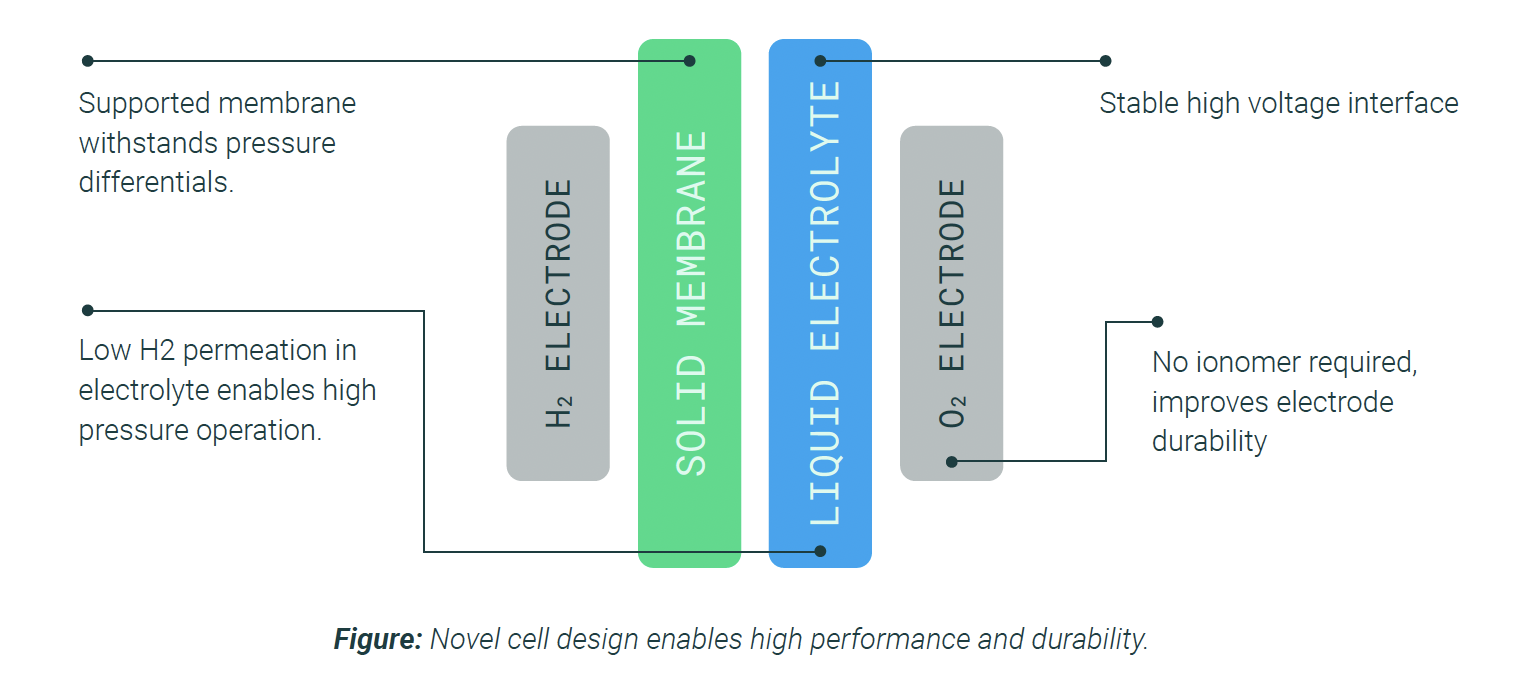
What makes AEM electrolysis revolutionary is its unique ability to deliver high efficiency while avoiding the use of precious metals, such as platinum or iridium. This positions it as a cost-effective alternative to PEM electrolysers while avoiding the complexity of traditional alkaline water electrolysis.
Unlike conventional alkaline electrolysers, which require concentrated potassium hydroxide (30% - 40% KOH) for ion conduction, AEM systems operate efficiently with dilute electrolytes (<1M KOH). This drastically reduces corrosion risks, maintenance costs, and safety hazards associated with handling caustic chemicals. At the same time, the solid anion exchange membrane enables stable operation at pressures of up to 30 bar, significantly exceeding the typical 3-bar limit of traditional alkaline systems. This makes compressing and storing hydrogen much easier and more efficient.
Power To Hydrogen leads innovation in AEM electrolysis technology by integrating the structural benefits of PEM with the material affordability of alkaline systems. Our membranes are engineered for enhanced electronic structure stability and low charge transfer resistance, enabling improved durability and performance.
To understand AEM technology fully, it's helpful to compare it with the two other primary hydrogen electrolysis methods:
Proton Exchange Membrane (PEM) electrolysers use a solid polymer electrolyte to separate and conduct protons between the anode and cathode. When water is fed to the anode side and a voltage is applied, it splits into oxygen, electrons, and hydrogen ions (protons). The protons migrate through the PEM to the cathode, where they combine with electrons to form high-purity hydrogen gas. PEM systems ramp from idle to full load in seconds, making them ideal for pairing with variable renewable power sources (such as wind and solar) and for applications that demand ultra-clean H₂ (e.g., fuel cells). However, PEM systems require expensive platinum group metals for operation, which result in higher upfront and operating costs.
Alkaline electrolysers utilize a liquid electrolyte consisting of potassium hydroxide (KOH) or sodium hydroxide (NaOH) to facilitate the movement of hydroxide ions (OH⁻) between electrodes. Applying voltage splits water into hydrogen at the cathode and oxygen at the anode, with hydroxide ions passing through the diaphragm to complete the circuit. As one of the oldest commercial water electrolysis methods, alkaline systems benefit from mature manufacturing and readily available materials. However, the system’s slower dynamic response makes it less ideal for highly variable renewable inputs.
AEM electrolysers provide an attractive balance between performance and affordability, validated by independent studies and real-world deployments. AEM stack costs are approximately 65% lower than those of PEM systems, while catalyst expenses are 75% lower. This significant cost advantage stems from two key factors: non-precious metal catalysts and standard manufacturing (simple stamped bipolar plates avoid the titanium machining required for PEM).
Note: $2.00/kg assumes renewable electricity at $0.03/kWh and 80% system efficiency. Costs drop below $1.50/kg with economies of scale.
.jpeg)
Cost Savings: AEM electrolysers use non-precious metal catalysts, such as nickel, which greatly reduces costs. These materials are much cheaper than the platinum-group metals used in PEM electrolysers and operate with benign liquid electrolyte solutions.
High Efficiency: By optimizing charge transfer and minimizing energy losses, AEM systems can achieve an efficiency of greater than 80% (HHV). High membrane conductivity enables efficient ion movement, thereby improving energy efficiency.
Longer Lifespans & Durability: AEM electrolysers offer fast reaction speeds and reduced corrosion issues, resulting in longer lifespans and enhanced durability. Improved catalyst layers help reduce energy losses and make the system more durable. That’s important because corrosion is a big issue in traditional alkaline water electrolysers.
Scalability: The modular design of AEM electrolysers allows easy scaling, from small test systems to large installations. Ideal for both decentralized and centralized hydrogen systems. When powered by solar or wind, AEMs support zero-emission hydrogen production.
Although AEM technology shows great promise, it still faces a few technical refinements as it scales toward commercial maturity. Overall, these limitations are more about refinement than roadblocks, manageable hurdles on the path to scaling a truly competitive emerging hydrogen technology. Room for improvement exists in the following categories:
Short device lifetime: One major issue is the limited understanding of how the key components degrade over time. These components, especially the membrane and catalysts, don’t last as long as those in PEM systems. This affects the overall stability and performance of the system.
While the aim is to surpass the lifetime of its competitors (PEM lasts 60,000-80,000 hours, and AWL lasts 80,000-100,000 hours). Most AEM electrolysers currently last less than 10,000 hours; however the improvements from Power To Hydrogen's patented AEM electrolysers have allowed it to match the durability of PEM and Alkaline electrolysers. (Based on 2023 IEC 62282-8-201 accelerated degradation tests at 1 A/cm², 60°C)
One area that is continually under research and development is membrane durability. Power To Hydrogen’s recent advances have improved performance, but some risk of electronic structure degradation remains, especially under fluctuating loads or high-temperature operation.
While lab-scale units are widely tested, large-scale deployments of anion exchange membrane water electrolysers are still in the process of maturation. Power To Hydrogen’s first pilot program was launched in 2024 and has since reached commercial maturity with stack deployments throughout the US and globally.
AEM electrolysers are becoming a powerful tool in the shift to hydrogen energy usage. Their modular design supports a wide range of applications, from fueling heavy mobility fleets to providing long-duration grid storage for intermittent solar or wind energy. Key use cases include:

AEM and PEM electrolysers both make hydrogen from water. But they work in different ways. AEM electrolysers use anion exchange membranes that conduct hydroxide ions (OH⁻), while PEM electrolysers use proton exchange membranes that conduct hydrogen ions (H⁺). AEM systems are generally less expensive and more adaptable to green hydrogen applications.
AEM systems vary widely, ranging from $5,000 for small pilot units to hundreds of thousands of dollars for industrial setups. The typical AEM stack cost is approximately 65% lower, and its catalyst cost is 75% less than that of PEM setups.
Power To Hydrogen’s AEM stack can produce hydrogen at $2.00 per kilogram, and in the future, the cost is expected to drop to $1.00 per kilogram within the next five years.
Modern AEM electrolysers operate at 80% efficiency (higher heating value). Research into better catalyst layers, electronic structure, and membrane durability continues to push these numbers higher.
As demand for green hydrogen grows across sectors such as transportation, chemical manufacturing, and energy storage, AEM electrolysers stand out as a versatile, cost-effective, and scalable solution. With ongoing improvements in membrane materials, catalyst efficiency, and system design, AEM systems are well-positioned to support the global transition to clean energy through sustainable hydrogen production.
Power To Hydrogen has played a key role in advancing AEM electrolysis as a practical option for clean hydrogen production. Our patented advancements continue in materials and system design to push AEM electrolysers forward by improving both affordability and performance.
For organizations seeking to produce hydrogen, install refueling stations, or develop long-term energy plans, Power To Hydrogen offers practical experience and ready-to-deploy systems that support reliable, clean energy outcomes.
Power To Hydrogen focuses solely on hybrid AEM electrolyser innovations. Our research and deployment partners include the Department of Energy, Shell, NASA, ARPA-E, multiple state universities, and commercial partners. Energy cooperatives, fleet operators, and Fortune 500 manufacturers choose us for transparent performance data, straightforward integration, and continuous improvement releases.
