


.jpeg)
Hydrogen electrolysis is a process that splits water (H₂O) into hydrogen and oxygen using an electric current. It’s one of the most promising ways to produce clean hydrogen, especially when the electricity comes from renewable sources. The process requires only water and electricity, making it a practical method for generating hydrogen without carbon emissions.
Electrolysis produces hydrogen for use in chemicals, fuel, metal processing, and long-term energy storage. Electrolysis allows for flexible production because the hydrogen electrolyser system can stop and restart quickly. If solar output dips, electrolysers can pause and resume when surplus renewable energy floods the grid. In this way, electrolysers act as controllable loads that stabilize frequency and reduce curtailment. Industries from food processing to steelmaking benefit from on-site hydrogen to replace natural gas or diesel.
In this guide, you will learn how hydrogen electrolysis technologies work, what drives their cost, and how our technology helps factories, freight fleets, and electric grids move toward net-zero goals without breaking their budgets.

Hydrogen electrolysis utilizes an electrolyser stack, which is a system comprising two electrodes, a membrane or electrolyte, and a power source to initiate the reaction. When electricity is applied, water molecules (H₂O) are broken apart. Hydrogen gas (H₂) is collected at the cathode, and oxygen gas (O₂) is released at the anode. This process produces high-purity hydrogen gas that can power fuel cells, feed industrial processes, or serve as a long-term energy storage source.
Effective water electrolysis depends on several factors. Cell temperature, water purity, and electrode coatings all affect the amount of electricity required to produce a kilogram of hydrogen. Even small gains in efficiency lead to significant cost savings at an industrial scale.
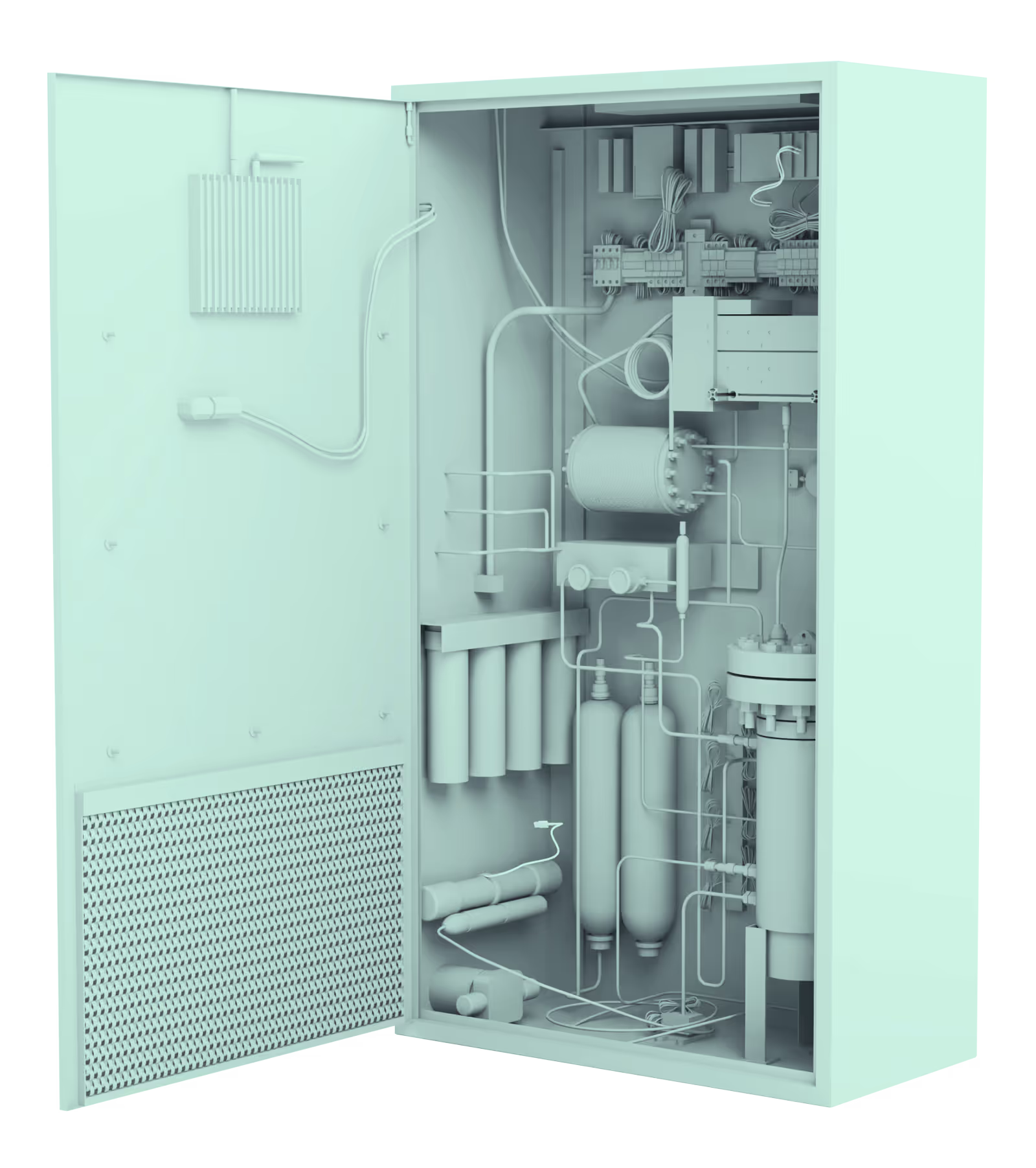
A commercial electrolyser is a complete system designed for precision, performance, safety, and integration. Key components include:
While most electrolysers operate on largely the same principles, there are a few functional distinctions that separate electrolysers into different categories.
Power To Hydrogen’s AEM (Anion Exchange Membrane) electrolysers blend PEM performance with alkaline cost advantages. They utilize non-noble catalysts and achieve stack efficiencies exceeding 80 percent (HHV). Modules scale from a few kilowatts for research labs to multi-megawatt arrays for industrial clusters. Three patented improvements have set our AEM electrolyser apart from other technologies that are on the market:

PEM stacks utilize a polymer membrane that conducts protons while blocking gases. Modern PEM systems achieve current densities above 2/A cm², attain 60–65 percent system efficiency (based on higher heating value), and deliver output pressures of up to 30 bar without mechanical compression.
Alkaline technology has supplied industrial hydrogen since the 1920s. In alkaline electrolysers, a porous diaphragm separates hydrogen and oxygen while a liquid potassium hydroxide electrolyte conducts ions. These devices tolerate more hours per year and offer lower capital cost per kilowatt than PEM stacks because they rely on plentiful nickel rather than platinum-group metals. Alkaline water electrolysers typically operate at 1.0–1.5 A/cm² and a 30–45% potassium hydroxide concentration, delivering hydrogen at atmospheric pressure.
SOEC units operate at 650–10 °C with a ceramic oxygen-ion–conducting membrane (yttria-stabilized zirconia). High temperatures slash electricity demand because heat supplies part of the reaction energy. When waste heat from a steel mill or cement kiln is available, SOEC efficiency can exceed 80 percent (higher heating value).
Breaking water into hydrogen and oxygen via electrolysis involves several key stages. A clear process flow ensures reliable output and predictable operating costs.
High-purity water is essential. Electrolyser cells demand water free of electrodes or minerals that could degrade membranes.
The electrolyte carries charged ions between the electrodes. In alkaline electrolysers, the liquid medium circulates outside the cells. In PEM and SOEC systems, the electrolyte sits inside the cell between electrodes. Maintaining the right electrolyte concentration and liquid medium temperature maximizes ion mobility and minimizes energy losses. Operators monitor pH and conductivity to keep conditions within narrow limits.
At the cathode, water molecules gain electrons to form hydrogen gas. At the anode, water releases electrons to form oxygen gas. The half-reactions are:
Modern control systems balance these reactions to avoid excess pressure or voltage spikes. Software tweaks current density in real time to extend electrode life and protect cell components.
After splitting, membranes prevent hydrogen and oxygen from mixing. Gas outlets collect each gas stream for piping or compression. Safety sensors watch for leaks and can trigger alarms within milliseconds. At client sites, we install fail-safe valves, allowing field teams to respond immediately to any abnormal readings.
Hydrogen production scales with stack size. Power To Hydrogen offers two modular systems:
Electrolysis offers a zero-carbon route when powered by renewable energy sources. Other methods of hydrogen production rely on fossil fuels or biomass. Here’s how electrolysis compares to other major production methods:
The most popular method of hydrogen production, steam methane reforming (SMR), accounts for 76% of global hydrogen output. SMR produces around 9 kg of CO₂ emissions per 1 kg of H₂ unless carbon capture and storage (CCS) is added to the system. Electrolysis powered by a 50 percent renewable grid already beats that footprint, and a fully renewable power supply removes it almost entirely. SMR remains the lowest-cost method today but fails to eliminate greenhouse gas release without CCS integration.
Levelized cost analyses from NREL in 2024 place alkaline electrolysis at $3 per kg in regions where wind delivers electricity at $20 per MWh. Inflation Reduction Act incentives (up to $3 per kilogram for zero-carbon hydrogen) further tilt the economics toward electrolysers, especially as carbon prices rise.
Hydrogen produced by electrolysis brings several clear advantages compared with fossil-derived hydrogen or other storage media.
These advantages to hydrogen electrolysis drive government incentives worldwide. The European Union’s Green Deal includes grants for electrolyser factories. The United States Inflation Reduction Act offers tax credits up to $3 per kilogram for hydrogen produced through electrolysis.

Electrolytic hydrogen is becoming a crucial component in the decarbonization of major industries. Many traditional sectors already rely on hydrogen, but the production method has historically been emissions intensive. With electrolysis, these same processes can continue without the carbon emissions.
These sectors often have continuous operations and significant power needs, making them ideal candidates for large-scale electrolysis paired with renewable power purchase agreements (PPAs).
Hydrogen can store excess renewable energy for days or weeks. Grid operators inject electrolytic hydrogen into salt caverns or steel tanks for seasonal balancing. When demand soars, the hydrogen feeds turbines or fuel cells that generate electricity. Electrolysers paired with wind and solar installations create hybrid energy systems with broader flexibility. Instead of wasting electricity during overproduction, excess energy gets stored in molecular form.
Hydrogen has a high energy density by weight, making it well-suited for long-range and high-duty-cycle applications. In these cases, the refueling time and total weight advantage offer a practical path that batteries can't meet. Hydrogen fuel-cell vehicles refuel in minutes and offer a greater range than battery electric vehicles of similar weight. Electrolytic hydrogen supplies dozens of stations worldwide.
Financial viability hinges on capital costs, operating expenses, and market dynamics. Tracking these factors guides investment decisions and project sizing.
Large central facilities benefit from economies of scale but require hydrogen transportation and storage infrastructure. Small-scale distributed hydrogen production facilities and electrolysers avoid transport but pay slightly higher per-kilowatt costs. Choosing between the two depends on end-use location, infrastructure availability, and project financing models.
Power supply drives 60 to 70 percent of operating expenses. Operators negotiate time-of-use rates or tap dedicated renewable projects for lower prices. Water treatment, maintenance, and stack replacements make up the remainder of total operating costs. AEM systems offer lower OPEX because they can use nickel instead of expensive materials required for PEM electrolysers.
The levelized cost of hydrogen (LCOH) is a metric used to assess long-term competitiveness, accounting for:
With current technology and renewable power at $20 USD per megawatt-hour, LCOH sits near $5.00 USD per kilogram for PEM and $4.00 USD per kilogram for alkaline. Real-world projects show Power To Hydrogen’s systems operating as low as $2.00 USD per kilogram.
New policies, such as the U.S. Clean Hydrogen Production Tax Credit (45V) and subsidies in the EU, are accelerating this transition by reducing the effective price gap. Similar premiums are also available in Germany’s H₂Global auctions and Japan’s Green Innovation Fund.
At Power To Hydrogen, we model cash flows for each client to identify grants, tax credits, and power purchase agreements that reduce effective hydrogen costs.
.jpeg)
Governments worldwide fund research to lower stack costs and improve efficiency. Bulk procurement contracts and incentive programs (such as the US IRA hydrogen production tax credit) sharpen commercial returns.
The United States Department of Energy’s Hydrogen Shot aims to achieve production at $1 USD per kilogram by 2030. Cost curves are moving quickly toward that target thanks to falling solar and wind prices and improved stack efficiency. If renewable power costs drop and electrolyser capital costs decline through mass production and innovation, these systems will reach the $1/kg target by 2030.
Cost remains a major factor in the adoption of hydrogen electrolysis. Building an electrolyser plant requires significant capital for stacks, power electronics, and balance-of-plant equipment. However, developers report that learning curves can halve equipment expenses after each doubling of cumulative production capacity.
Efficiency also demands attention. Conventional electrolysis systems convert around 65 percent of input electricity into stored hydrogen energy. Heat generated during operation must either be dissipated or captured to boost net efficiency. To improve efficiency, research teams at Power To Hydrogen focus on advanced catalyst coatings that lower cell overpotentials.
Materials durability represents another hurdle. Membranes and electrodes degrade over time when exposed to impurities or uneven current distributions. Greater wear leads to more frequent stack replacements. Power To Hydrogen is addressing this challenge by enhancing membrane chemistry and introducing hybrid alkaline solutions.
Scaling up supply chains also matters. Precious metals such as iridium and platinum serve as catalysts in PEM electrolysers. Global reserves of these metals are limited, and demand may outstrip supply if hundreds of gigawatts of capacity come online. To address this, Power To Hydrogen’s AEM electrolyser stacks use non-precious metal catalysts based on nickel alloys.
Policy support accelerates progress. Grants, tax credits, and low-interest loans help close the cost gap between green hydrogen and fossil-derived alternatives. Programs in Europe and North America now fund pilot projects that demonstrate full supply-chain integration, from renewable energy generation to end-use applications. Those real-world cases build confidence among investors and industrial end users.
Power To Hydrogen’s electrolysers are already deployed across multiple pilot and commercially operated industrial sites. In one project, we partnered with a utility to provide hydrogen generation for grid support during periods of solar overproduction.
Industrial pilots, such as the 2025 AEM installation at the Port of Antwerp-Bruges, demonstrate reliable operation on a large scale. Each deployment teaches us more about real-world integration, and we bring those insights to new clients to shorten the development curve.
Power To Hydrogen ships 10-kW (K-Class) and 250-kW (M-Class) containerized skids that stack like server racks. Our electrolysers can be run in parallel to form up to a 25-mW block. This building-block strategy allows phased capital spending and immediate power generation on the first installation.

Most commercial systems need fifty kilowatt-hours per kilogram today. Development targets forty-five kilowatt-hours within five years.
Electrolysers paired with pressure-swing adsorption or membrane dryers achieve ultra-high purity (up to 99.999%), making them suitable for fuel-cell vehicles and semiconductor fabs.
The process consumes about nine kilograms of water for each kilogram of hydrogen. Closed loops reclaim condensate, keeping net demand low.
Ideal feed water meets ASTM Type II standards or better. That means total dissolved solids below 1 part per million. Pre-treatment systems remove ions and dissolved oxygen to protect membranes and catalysts.
Typical stack lifetimes range from 60,000 to 80,000 operating hours under steady conditions. Routine maintenance may include replacing membranes or cleaning electrodes. With proper operation, a system can run for a decade before requiring major stack refurbishment.
System sizing depends on hydrogen demand, available power, and desired pressure. Small refueling stations may require a capacity of 0.5 to 2 megawatts. Industrial ammonia plants often deploy tens to hundreds of megawatts of power. Our engineers work with clients to model production profiles and recommend optimal stack counts.
Modular electrolyser designs allow rapid capacity additions. Once foundations and power connections are in place, adding each new 1-megawatt container usually takes a few weeks. In contrast, building a greenfield plant with multiple stacks might span several months.
Power To Hydrogen offers Electrolysers as modular units in two models, the 10-kW K-Class and the 250-kW M-Class. The electrolysers are designed to be run in parallel with repeated units capable of generating up to 25-mW. Smaller units are suitable for research labs or remote sites. Larger megawatt-scale installations serve industrial parks and hydrogen hubs.
It typically takes 50-55 kWh of electricity to produce 1 kilogram of hydrogen using electrolysis, depending on the system efficiency.
